Producing an effective and safe vaccine is laborious, and the risks taken in the race to discover a vaccine against COVID-19 are extremely high. With billions of dollars pledged globally, scientists are working to cut the development time of a COVID-19 vaccine. Studies suggest that it takes an average of 17 years for research evidence to reach clinical studies. In the COVID-19 pandemic such time-lags are impossible. Research that would normally take years is now being completed in months. Scientific experts that are most knowledgeable about vaccine development and the real dangers of reckless haste warn that however promising a vaccine may seem now or months from now, premature release can do far more harm than good.
Vaccinations have reduced disease, disability and death from measles, polio and smallpox. The scientific and vaccine community have responded urgently to epidemics like Ebola, H1N1 influenza, Zika and now SARS-CoV-2 and know from experience that vaccines may sometimes seem promising in vivo and during early trials but fail to perform in the real world. For example, in 1955 the original Salk polio vaccine was hastily rolled out. A mishap in mass-producing the vaccine caused polio in 70,000 children, permanently crippling 164 of them and killing 10. As recent as 2016, Dengavaxia was intended to protect children from the dengue virus but instead it increased the hospitalization rate for children who received the vaccine compared to unvaccinated participants.
Among the top concerns is the potential that a fast-tracked COVID-19 vaccine will have unintended side-effects. No vaccine is 100% effective and safe, but if 1 billion people are vaccinated, a 1 in 10,000 serious adverse events will affect 100,000 of those people. In May 2020 it was revealed that 4 out of 45 participants in Moderna phase 1 vaccine trial experienced “medically significant” adverse events.
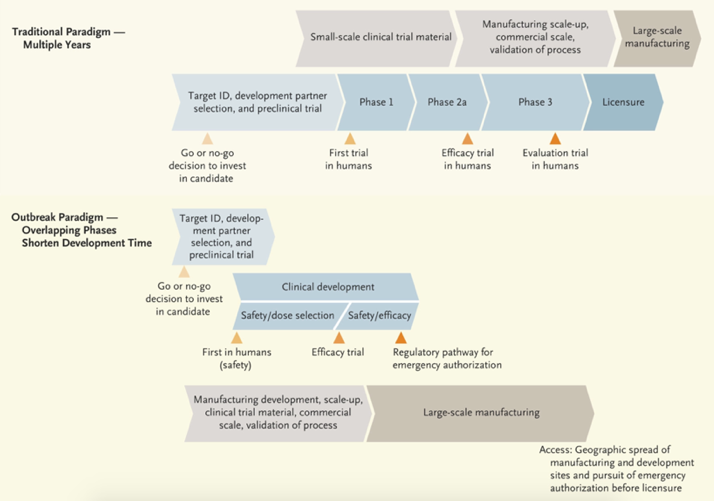
Traditional vaccine development vs. using a pandemic paradigm
Developing a vaccine quickly requires a pandemic paradigm, with fast starting and numerous steps executed in parallel before confirming a successful outcome, hence exposing all of us to unnecessary dangers. The pandemic paradigm is conducted without knowing whether the vaccine candidate will be safe and effective. The race for a vaccine may lead to a product that is scaled up to commercial production before the demonstration of clinical efficacy and safety.
Apart from safety issues, some vaccines worsen the consequences of infection rather than protect, a phenomenon called antibody-dependent enhancement (ADE). ADE is a well recognized phenomenon whereby viral entry into cells is enhanced by binding of virus/antibody complexes to membrane receptors, resulting in worsening of disease by encouraging the virus into cells to replicate, exacerbating the disease. ADE was recently thrust into the spotlight with Dengue fever and has also been observed in other clinically relevant viruses like HIV and Ebola. This phenomenon has also been observed in previous attempts to develop coronavirus vaccines. More concerning is the presence of antibodies typical to ADE in the blood of some COVID-19 patients. Researchers believe that there is definitely a risk of ADE, yet the US National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center, which is collaborating on Moderna’s vaccine, has downplayed the possibility of ADE in a COVID-19 vaccine.
According to surveys in the United States, approximately 30% of the public say they would reject a COVID-19 vaccine. Even if a vaccine proves to be safe and effective, it won’t do much good if many people refuse it. Ensuring a vaccine is safe and effective will be essential in keeping the public’s trust in vaccines.
 With struggling economies around the world and governments pilling up millions of dollars in debt, there is no doubt that we are in urgent need of a COVID-19 vaccine that works. Rolling out a COVID-19 vaccine too quickly could be dangerous, because there is no way of knowing how long any vaccine would be able to protect our population from this virus. Testing COVID-19 vaccines without taking the time to fully understand safety risks could bring unwarranted setbacks during the current pandemic and in the future. Vaccine scientists and manufacturers need to carefully assess the type of immune response the vaccines induce and test the response in vaccinated animal models exposed to the virus to ensure ADE does not occur. The FDA must also apply rigorous standards when considering whether to authorize a coronavirus vaccine.
With struggling economies around the world and governments pilling up millions of dollars in debt, there is no doubt that we are in urgent need of a COVID-19 vaccine that works. Rolling out a COVID-19 vaccine too quickly could be dangerous, because there is no way of knowing how long any vaccine would be able to protect our population from this virus. Testing COVID-19 vaccines without taking the time to fully understand safety risks could bring unwarranted setbacks during the current pandemic and in the future. Vaccine scientists and manufacturers need to carefully assess the type of immune response the vaccines induce and test the response in vaccinated animal models exposed to the virus to ensure ADE does not occur. The FDA must also apply rigorous standards when considering whether to authorize a coronavirus vaccine.





Join the Conversation